
“Today’s compound makes no noise and leaves no wreckage. It merely stinks. But it does so relentlessly and unbearably. It makes innocent downwind pedestrians stagger, clutch their stomachs, and flee in terror. It reeks to a degree that makes people suspect evil supernatural forces. It is thioacetone.”
— Dr. Derek Lowe, Science Journal
In his personal journal Meditations, the philosopher-king Marcus Aurelius kept level-headed by reminding himself the purple of his robes came from dead shellfish. Well, marine snails to be more exact. The city of Tyre turned capturing this precious color into a literal art form.
The snails were taken by the thousands and boiled in a led vat for days, producing a smell which was royal in no way. But at least you got something exotic out of it, like purple.
Thioacetone gives us nothing. According to scientist Dr. Derek Low, it just reeks to a degree not thought possible.
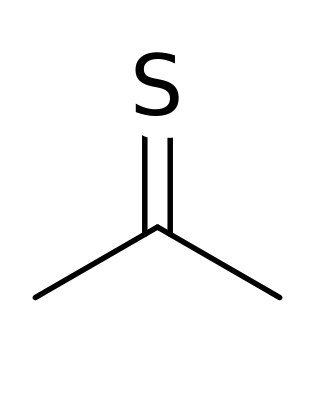
And the substance by its very nature almost doesn’t want to exist. It’s inherently unstable. The sulfurous compound turns into a polymer if temperatures rise above -20C (-4F). But real trouble begins when Thioacetone sticks around and someone tries to break it open.
Dr. Low says the real cause of the terrible odor is hard to isolate because “when people start diving out of windows and vomiting into wastebaskets… the quality of the data starts to deteriorate.”
Obviously, people have a tendency to exaggerate but thioacetone has some street credit to back up its smelly reputation.
Backing Up Its Odorous Infamy
Perhaps the ultimate stink story for thioacetone involves an 1889 experiment in a lab at Freiberg, Germany. A reaction with the substance caused an odor which leaked out of the testing area and resulted in evacuations and mass sickness within the city.
Randall Munroe at the New York Times says the smell spread a half mile in all directions. This wasn’t a large amount of it either. While the various articles I’ve researched never say exactly how much thioacetone caused the odor, it leans more towards a beaker than a vat.
In 1890, Dr. Lewkowitsch in The Chemical News mentions thioacetone and rose oil have a similar property: dilute them with air and their scent gets more powerful. While rose oil is pleasant, the former in Lewkowitsch’s own words becomes “fearful.”
He mentions experimenting with the substance and having workman from the opposite end of the steelworks where his lab was located, tell him they were too sick to continue their job. So, thioacetone appears to have the odd ability to accumulate more punch as it travels.
Dr. Lowe also mentions experiments done in the 1960s at Esso Research Station in Abingdon, UK. During one mishap, a stopper popped off a bottle. Despite the top being replaced instantly, “an immediate complaint of nausea and sickness from colleagues working in a building two hundred yards away,” came trickling in.
In fact, the scientists found tiny amounts of thioacetone could be detected up to a quarter mile away “in seconds.” Although scientists near it weren’t aware.
After practice the team at Esso found the following protocol had to be followed when working with the substance to contain the odor:
“The offensive odors…are confined and eliminated by working in a large glove box with an alkaline permanganate seal, decontaminating all apparatus with alkaline permanganate, eliminating obnoxious vapors with nitrous fumes generated by a few grams of Cu in HNO3, and destroying all residues by running them into the center of a wood fire in a brazier.”
So, by now I’m sure you get the point: thioacetone stinks. And something with such an odor must be useless. Well, not exactly.
What A Properly Used Bad Odor Can Do
Randall Munroe reminds us Ethyl mercaptan is added to natural gas to give it that funky rotten egg smell. Without the additive, it’s odorless. Therefore, the stink enables us to easily detect a leak.
But David Hambling at The Guardian has a more malevolent use: crowd suppression. He describes the US Army’s XM1063 project. Although details are sketchy, it appears to comprise of a shell fired from a howitzer that parachutes down and sprays various chemicals.
One of the possible choices appears to be a malodorant or as Hambling describes it “a super stink bomb.” What’s more, a presentation from the US Army Field Artillery Center confirms this. In their slides they say:
“Artillery delivered malodorants will disperse crowds and separate combatants from noncombatants, thereby reducing the number of civilian casualties at the objectives.”
Hambling says this is tiptoeing around the edge of the Chemical Weapons Convention. Although some think it crosses the line. Then again, what if you didn’t have to spray a crowd with chemicals?
Imagine opening a beaker and a smell wafting for half a mile powerful enough to make a mob run in another direction. Now, that’s worthy of being called “fearful.”
Hopefully, thioacetone remains one of those lab curiosities and nothing more.
-Originally posted on Medium 3/11/23


